A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. As electrons are added, they fill electron shells in an order determined by which configuration will give the lowest possible energy. The first shell (n=1) can have only 2 electrons, so that shell is filled in helium, the first noble gas. In the periodic table, the elements are placed in 'periods' and arranged left to right in the order of filling of electrons in the outer shell. So hydrogen and helium complete the first period.
- Lewis Dot Structure For Oxygen
- Lewis Dot Structure For Ch4
- Lewis Dot Structure For Nh3
- Lewis Dot Structures
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the. . Electrons in a single Lewis structure are assigned to specific atoms-a single Lewis structure is insufficient to show electron delocalization. Composite of resonance forms more accurately depicts electron distribution. Why Write Resonance Structures?
The number of electrons in a given shell can be predicted from the quantum numbers associated with that shell along with the Pauli exclusion principle. The second shell, associated with principal quantum number n=2, can have a maximum of 8 electrons and corresponds to the second period of the periodic table. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them.
Lewis Dot Structure For Oxygen
| Lewis Dot Diagrams | Visualization of electron orbitals |
- A Lewis structure is a structural representation of a molecule where dots are used to show electron positions around the atoms and lines or dot pairs represent covalent bonds between atoms. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation.
- Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. Use Lewis structures as a guide to construct three-dimensional models of small molecules. Determine the electron and molecular geometry of the produced molecules. Background: Scientists often create models to represent either a physical.
A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as 'dots' or for bonding electrons as a line between the two atoms. The goal is to obtain the 'best' electron configuration, i.e. the octet rule and formal charges need to be satisfied.
Note
Lewis structure does NOT attempt to explain the geometry of molecules, how the bonds form, or how the electrons are shared between the atoms. It is the simplest and most limited theory on electronic structure.
How to draw Lewis Diagrams
Lewis Dot Structure For Ch4
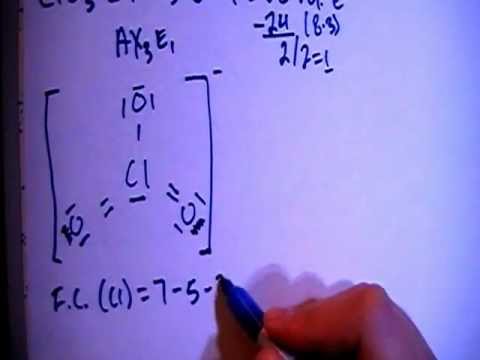
The following is an example of how to draw the 'best' Lewis structure for NO3- (learning by example).
- First determine the total number of valence electrons in the molecule. This will be the sum of the group number a of all atoms plus the charge.
| N | 5 |
| O (x 3) | 18 |
| charge | 1 |
| 24 |
- Draw a skeletal structure for the molecule which connects all atoms using only single bonds. The central atom will be the one that can form the greatest number of bonds and/or expand its octet. This usually means the atom lower and/or to the right in the Periodic Table, N in this case.
- Now we need to add lone pairs of electrons. Of the 24 valence electrons available in NO3-, 6 were used to make the skeletal structure. Add lone pairs of electrons on the terminal atoms until their octet is complete or you run out of electrons.
- If there are remaining electrons they can be used to complete the octet of the central atom. If you have run out of electrons you are required to use lone pairs of electrons from a terminal atom to complete the octet on the central atom by forming multiple bond(s). In this case the N is short 2 electrons so we can use a lone pair from the left most O atom to form a double bond and complete the octet on the N atom.
- Now you need to determine the FORMAL CHARGES for all of the atoms. The formal charge is calculated by: (group number of atom) - (½ number of bonding electrons) - (number of lone pair electrons), i.e. see the figure below.
No Lewis structure is complete without the formal charges. In general you want:
- the fewest number of formal charges possible, i.e. formal charges of 0 for as many of the atoms in a structure as possible.
- the formal charges should match the electronegativity of the atom, that is negative charges should be on the more electronegative atoms and positive charges on the least electronegative atoms if possible.
- Charges of -1 and +1 on adjacent atoms can usually be removed by using a lone pair of electrons from the -1 atom to form a double (or triple) bond to the atom with the +1 charge. Note: the octet can be expanded beyond 8 electrons but only for atoms in period 3 or below in the periodic table. In our present example N can not expand beyond 8 electrons so retains a formal charge of +1, but the S atom below can expand its octet.
- You have determined the 'best' Lewis structure (octets completed and lowest formal charges) for NO3-, but there are a number of ways to show this structure. Although it is most common to use a line to indicate a bonding pair of electrons they can be shown as electrons, see the left most image below. It is also common to show only the net charge on the ion rather than all of the formal charges, i.e. see the right most figure below.
Lewis Dot Structure For Nh3
Why are there different ways for the 'same' Lewis structure? It depends what you want to show. While the most complete structure is more useful for the novice chemist, the simplest is quicker to draw and still conveys the same information for the experienced chemist. You should learn to recognize any of the possible Lewis structures.